This is a looooong article about the world's second best selling hair loss treatment product Finasteride. For regular customers, it will be more than enough to just read the 16 frequently asked questions about finasteride. If you want to be an expert on finasteride, you can read the second part, which is our professional finasteride review.
1. What is Finasteride?
Finasteride is one of the two FDA approved products for hair loss treatment, the other being minoxidil. Interestingly, both finasteride and minoxidil were discovered to have hair growth effect as side effects. Minoxidil was found to grow more hair in those people who took in as an antihypertensive vasodilator medication; while Finasteride (brand name Propecia) was found to halt hair loss and even reverse hair loss when it was taken to treat benign prostatic hyperplasia.
2. How does finasteride work?
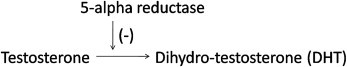
Finasteride inhibits expression of the enzyme, 5-alpha reductase, which regulates production of dihydrotestosterone (DHT). By lowering DHT levels in the scalp, it reduces DHT's harmful effect on hair follicles. Finasteride decreases DHT concentrations in the serum and the scalp by up to 70 and 60%, respectively. Male pattern hair loss, or androgenetic alopecia, is an androgen-dependent disorder. In genetically susceptible men, dihydrotestosterone (DHT), a potent metabolite of the male androgen testosterone, contributes to male pattern hair loss. The conversion of testosterone to DHT is regulated by the enzyme 5-alpha reductase.
3. How does finasteride affect the body's hormonal / endocrine system?
Finasteride (Propecia® and others) is a specific type II 5-alpha reductase inhibitor (on the contrary, Azelaic acid is a non-specific 5-alpha reductase). That is, it inhibits the enzyme responsible for regulating conversion of testosterone to dihydrotestosterone (DHT). By reducing DHT levels in the scalp, the drug decreases DHT's effects on the hair follicles, reversing the process of hair loss.
4. Is topical finasteride as effective as Finasteride pill?
Finasteride is initially approved as oral pill to be taken once a day. The usual dose is 1mg daily taken with or without food, at any time during the day. A topical formulation of finasteride has been approved in India and has been found to be as effective as oral finasteride.
5. Why women should not use finasteride?
Finasteride is not indicated for use in women with hair loss (female pattern hair loss). Finasteride is contraindicated in women when they are or may potentially be pregnant, because it may cause abnormalities of the external genitalia of a male fetus (Pregnancy Category X, i.e., drugs that have such a high risk of causing permanent damage to the fetus that they should not be used in pregnancy or when there is a possibility of pregnancy.)
6. How effective is finasteride?
The data are from three large, multicentre, placebo-controlled studies of 1,879 men with mild-to-moderate, but not complete, male pattern hair loss. The men received either oral finasteride once daily or placebo for one year. The endpoints for the studies were objective hair counts taken from a 1-inch diameter circular area, and subjective assessments of improvement by patients, investigators, and an independent panel of dermatology experts who evaluated pre- and post-treatment photographs.
The trials showed that finasteride can prevent hair loss in men with mild-to-moderate male pattern hair loss. In two of the clinical studies involving men with mild-to-moderate male pattern hair loss, 86% of men treated with finasteride maintained or showed an increase in the amount of their hair based on hair counts during the course of the studies. Only 14% of men treated with finasteride had further hair loss after 12 months of treatment, compared with 58% of placebo patients.
On-going studies have demonstrated that finasteride halts hair loss or regrows hair in 9 out of 10 men taking it long term every day.
7. Are some patients more likely to respond to finasteride than others?
It is difficult to predict which patients will respond and to what extent. Patients studied in clinical trials had mild-to-moderate, but not complete, hair loss, and ranged in age from 18 to 41.
8. How long do I need to take finasteride to see any result?
Daily use for three months or more may be necessary before a patient will notice prevention of further hair loss or increased hair growth.
9. Can I stop use finasteride once I have grown enough hair?
Daily use of finasteride for three months or more may be necessary before a patient will notice prevention of further hair loss or increased hair growth. There are no controlled clinical data on treatment for longer than 12 months. It probably will be necessary to continue treatment indefinitely to maintain the benefits.
10. What happens if you stop taking finasteride?
If a patient stops taking finasteride, DHT levels will rise again in the scalp, and it is likely that hair loss will resume.
11. Will I grow more hair on other part of my body?
Finasteride is a specific inhibitor of type II 5-alpha reductase, which is found primarily in scalp hair follicles. In clinical trials, there was no reported effect on hair on the other parts of the body.
12. What are the side effects of finasteride?
In clinical trials, finasteride was very well tolerated in men, with most patients reporting no serious side effects. The principal side effects associated with finasteride were decreased libido (1.8% of finasteride patients versus 1.3% on placebo) and erectile dysfunction (1.3% finasteride versus 0.7% placebo). In addition, decreased volume of ejaculate was reported in 0.8% of men treated with finasteride and 0.4% of those on placebo. Gynaecomastia (breast enlargement) has also been reported. All of these side effects resolved upon discontinuation of therapy, and also resolved in many men who preferred to continue therapy. Postmarketing reports have included some patients complaining of depression, but the risk of this is likely very small. See more detailed information here.
13. What is the long-term effect of using finasteride?
Finasteride was evaluated in 3,200 men and it was very well tolerated, including patients on therapy for up to two years. Long-term suppression of DHT does not appear to be harmful. This is based on extensive research, dating back to 1974, of men born with a deficiency of 5-alpha reductase.
Finasteride results in an average reduction of 50% for the prostate specific antigen score (PSA test). The score should be doubled to work out the risk of prostate cancer. The risk of prostate cancer related to finasteride use has been very carefully reviewed. Current evidence is reassuring and it does not seem to increase the risk or cancer nor result in more serious disease than in those that do not take the drug. Although some cases of breast cancer have been reported in patients on finasteride, there is no evidence that the drug was the cause.
14. Are there any contraindications for finasteride?
Finasteride is not indicated for use in women or children. However, it is sometimes prescribed off-label for postmenopausal women without problems. Finasteride is contraindicated in women who are or may potentially be pregnant. Finasteride is contraindicated in patients who are hypersensitive to any component of the product.
It is not recommended in men that are subfertile. In men planning to have children, some doctors check sperm counts prior to starting finasteride and repeat it after 6 months of treatment. If the sperm count has reduced, finasteride should be stopped.
15. Does finasteride cause any interactions with other drugs?
No drug interactions of clinical importance have been identified. Studies have been conducted with finasteride and antipyrine, digoxin, glyburide, propranolol, theophylline, and warfarin, and no interactions were found. In clinical trials, finasteride was used concomitantly with ACE inhibitors, acetaminophen, alpha blockers, benzodiazepines, beta blockers, calcium channel blockers, cardiac nitrates, diuretics, H2 antagonists, HMG-CoA Reductase inhibitors, prostaglandin synthesase inhibitors (NSAIDs), and quinolones, without evidence of clinically significant adverse interactions.
16. Can you combine finasteride with minoxidil?
The combination has been shown to offer better results than minoxidil or finasteride alone. If switching from one treatment to the other, it is best to overlap by six months to reduce chance of hair loss from withdrawal of the fist agent while waiting for response to the new product.
Want to be an Expert on Finasteride? Here you go...
Hippocrates was the first to study and understand the relationship between baldness and the levels of testosterone in an individual. In his study, it was observed that male eunuchs, mostly who were young, showed no signs of hair fall or hair loss. If there is a genetic deficiency in men, especially the second iso-enzyme of 5- α Reductase, which results in no hair fall and no baldness patterns being caused. It can mainly be attributed to the fact that testosterone is converted into Dihydrotestosterone (DHT) by the I and II Reductase. The Type I Reductase is found to exist abundantly in the skin, which includes the scalp, whereas Type II is prominent in the prostate gland and hair follicles. It can be seen in the figure how a finasteride molecule inhibits the Type II 5- α Reductase and does the work. Due to the inhibition caused, the serum level of DHT present in the scalp is reduced and subsequently, results in increased scalp levels in the testosterone. An illustration of the same can be seen in the figure on how finasteride affects both.
DHT, Dihydrotestosterone
The effects on DHT and testosterone levels by finasteride or placebo dosages of 5mg on a daily basis were studied in a set of 17 patients. These patients had undergone the procedure of scalp biopsy to test the concentrations of DHT and testosterone for 28 days. The results showed that the level of DHT present was higher in the areas that went bald and the presence of testosterone did not vary significantly. Also, patients who were subject to treatment by finasteride showed a decrease in the concentration of DHT as it dropped to 3.62 pmol/g from the previous level of 6.4 pmol/g. There was an increase in the testosterone levels in 6 out of 8 patients when were taking finasteride. The mean DHT concentration came down to 0.46 nmol/L from 1.36 nmol/L after a 28 day of subjection to finasteride and the testosterone levels present in the serum remained the same.
When the study was conducted on a larger group of 249 patients, the dosage was randomized and it varied from 0.01 to 5 mg/day and the motive was to understand the precise dosage at which there were visible and evident effects on the DHT levels of serum and scalp. The results were surprising, as results were seen in individuals who were subject to a meagre dosage of .2 mg/day and the decrease in scalp DHT levels varied from 60% to 75%. The testosterone levels in the serum did not bear any change on the ongoing balding process and as the study concluded, it was decided with adequate studies that the dosage for AGA was supposed to range from 0.2mg to 1mg every day.
On completion of this study, AGA has ideally recommended a dosage of 1 mg/day and eventually went ahead to obtain an approval from FDA for this product and successfully obtained it in 1997. The commercial name used was Propecia which was marketed by Merck & Co Inc, Whitehouse Station, NJ. Also, based on the study, it was discovered that finasteride underwent metabolism to a great extent in the liver. Thus, patients who have abnormal functioning of the liver should consumer this under proper medical guidance. It does not interact with any other drug as per the findings the drug metabolizing enzyme named the cytochrome P450 was left unaffected. Also, this drug did not have any side-effects or issues when used on people who were administered with warfarin, propranolol, theophylline, digoxin or any anti-pyrite drugs.
Efficacy and Safety in Men
There were numerous studies performed to study about the hair growth particularly in the vertex area when men were administered with finasteride. The study period went up to one year and on a random basis, 1553 men were subject to a constant dosage of 1 mg placebo or 1 mg finasteride daily. Following that was a blinded extension for the second year and during this period, people administered with finasteride showed a significant increase in the hair count in the area of the previously balding vertex in comparison to those administered with placebo. Also, when the trail period was extended to five years, during the study period, it was found that the growth was at its peak during the period of first to second year and it remained well above the baseline for 90% of the individuals who were being tested.
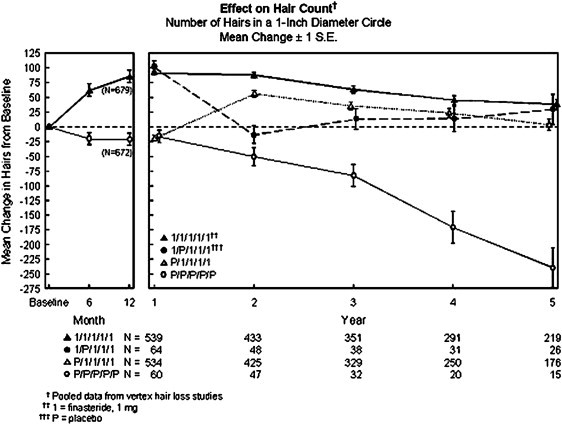
In a trail which was performed by controlling the placebo and used 1 mg finasteride daily, it was found that anterior and mid-scalp hair loss was also treated with efficacy. To back these findings, there were studies from laboratories that were performed by administering 5 mg of finasteride to stump-tailed macaques, both male and female. The weight of the hair and the mean follicle length which was measured along the frontal scalp was found to increase. The AGA demonstrated in a random and double-blind placebo controlled trail which was conducted on 9 sets of twins, who were males, that the individuals who were subject to 1 mg/day of finasteride for one year had comparatively better hair growth than the perfectly matched controls, proving it to be useful even in the process of hair transplantation for setting the hair. In yet another trial that was conducted, 79 men were given 1 mg finasteride or placebo daily for a period of 4 weeks prior to hair transplantation and up to 48 weeks post the hair transplantation procedure with a double-blind AGA trial. The results pointed that there was better level of improvement in individuals with finasteride intake rather than placebo.
Even though there is a great impact of using finasteride, it is a great idea to actually know about how it works and the mechanism involved in hair follicles. The study trail was conducted on 212 men who had AGA and they were randomly given samples of finasteride and placebo with the dosage of 1 mg/day for a period of 48 weeks to check for the results in the anagen to telogen ratio in hair. The long-term studies were conducted and it was concluded that there was an increase in the weight of hair for a sprawling span of 3 to 4 years. There was a greater level of weight gain in the hair when compared to the hair count and it was proven again that finasteride promotes the length of hair growth and also the hair thickness. Within a period of 192 weeks, when finasteride was used, there was an increase of 21.6% in comparison to the baseline and in contrast, there was a decrease of 24.5% in comparison to the baseline when placebo was used. Similarly, in the hair count level, there was a 7.2% increase from baseline by using finasteride whereas there was a 13.0% decrease from the baseline in the case of placebo.
Efficacy and Safety of Women
Female at childbearing age cannot use finasteride unless strict and stringent birth control measures is used, because finasteride is classified as category X for pregnancy. The main reason behind this is because feminization can be resulted in male fetus due to the usage of finasteride. Due to this, women are advised to stay away even from broken or the handle-crushed pills. The effects of finasteride have not been known on children and males can continue using it even when their spouse becomes pregnant. Apart from the risk involved in pregnancy, there are no considerable effects on the hair fall in women.
There was a trial conducted among 137 postmenopausal women and they were subject to 1 mg of either finasteride or placebo. In the double-blind trail, despite a prolonged study period and careful examination, there was no effect in stopping hair thinning, or a difference in the appearance of hair among the group that was treated with finasteride.
However, finasteride was beneficial for women who were having hyperandrogenism. Due to this, it was concluded that the reasons for hair loss vary among women and the need for a study with more controlled trials and investigation was needed to study the effect of finasteride. The trail group should be divided into 3 sub-groups, one which was treated with finasteride and oral contraceptive pills, Exclusively OCP and the third group only with placebo, as per the findings. However, the study is yet to be undertaken.
In a separate study that was conducted in Italy, it was found that there was an increase of 23-27% among the premenopausal normoandrogenic women who consumed 2.5mg of finasteride in addition to OCP that comprised of drospirenone and ethinyl estradiol. Despite the results being successful, the antiandrogenic effects arised due to birth control still exist due to the increased dosage of finasteride. It was concluded that women who had an increased dosage of oral finasteride up to 5 mg, benefitted from it. It was also proposed that there might be a possibility of women having increased activity of 5- α Reductase enzyme due to which they do not improve when subject to systemic antiandrogenic treatments but however, showed signs of improvement when finasteride was used.
Effects on prostate specific Antigen Levels
The prostate-specific antigen (PSA) score was found to decrease up to an extent of 50% by administering finasteride. To determine this, there was a study conducted on 355 men aged between 40-60 years and finasteride and placebo were given to individuals in a ratio of 4:1. The median decrease in serum PSA in the age group of 40-49 showed 40% decrease whereas in the age group of 50-60, the decrease exhibited was 50%. The tests were conducted by administering a dosage of 5 mg/day.
The sensitivity to PSA also stands increased in screening and the curve of PSA was found to be greater in the group with finasteride when compared to placebo. Due to this, better detection and diagnosis of prostate cancer is possible.
Risks of Prostate Cancer
The impact of finasteride has been studied to know more about the rate and grade of impact on prostate cancer. There was a trail conducted known as the Prostate Cancer Prevention Trial (PCPT) and the sample considered was of 18,882 people aged 55 years with a variation of 7 years and the emergence of prostate cancer went down by 24.8% among people who consumed 5 mg of finasteride instead of placebo daily. Tumors that have Gleason scores ranging from 7-10 is more prevalent among people administered with finasteride group was found to more frequent. This trial was conducted on an older age group and has proved to be that finasteride is a chemopreventive agent.
Also, it has been found that histomorphologic changes are not induced due to finasteride in prostatic carcinoma. The histopathologic differences that arise in carcinoma of placebo and finasteride were not distinguishable. There are chances of loss of luminal glandular space and the infiltration among single-cells, due to which cancer of high-grade is caused. It is also used in androgen deprivation therapy.
Detection bias can be used to examine the increased number of high-grade cancer occurrences. The relative size of core biopsy is increased as there is a reduction in the volume of prostate volume. Due to this, the sensitivity of biopsy is increased and the probability of finding diseases of high-grade are significantly high. Cancer detection chances are increased by 23% when the prostate volume is decreased by 25%. Smaller prostates were found to have a greater chance of high-grade cancer occurrence and based on this, it could be concluded that there were no effects of finasteride whatsoever on cancer.
Effects on Sperm
As per the various studies conducted, there were no effects whatsoever on the sperm count. The largest study conducted in association with AGA, comprised of 181 men in double-blind and was a randomized study who were given 1 mg of finasteride or placebo for a span of 48 weeks and towards the end, it was concluded that there were no effects on total sperm ejaculation, sperm motility or the sperm morphology. It was also concluded that testosterone is the primary androgen that is responsible for regulating spermatogenesis, sperm maturation and seminal fluid in testis, epididymis and seminal vesicle but not the DHT. However, in a recent study, the sperm count was found to have dropped by 34% when an individual was given 5 mg/day for a span of 26 weeks. But after the 26 weeks window, there were no changes at all when the study was conducted up till the 52nd week.
In yet another study, three men were given 1mg /day of finasteride for 5 years and their sperms were analyzed. The sperm morphology was found to be consistent with necrosis when it was observed under an electron microscope. The motility was found to have reduced to a great extent among 2 of the men and one of the individual was an azoospermic. Also, after a period of one year, the spermatogenic process found to be stood improved.
Severe azoospermia was observed in two patients and the result was them being infertile. Due to this, it can be concluded that finasteride needs to be stopped if there needs to be an improvement in semen parameters for couples wanting to have babies.
Side Effects
Apart from the risk of feminization in a male fetus, the reports of sexual side-effects have been ambiguous. 2% of the males claim to have had an effect on the sex drive, which included decreased libido, erectile dysfunction and ejaculation disorder which was found to be at a lower rate of 1% among the placebo group. There was an incidence rate of .4% for Gynecomastia, mastalgia and it did not occur until the completion of treatment period. There were also cases of exfoliative dermatitis, perioral numbness, swollen glands which could be cured by drug cessation. The adverse effect of finasteride is not a direct result of pharmacologic action but is induced when the patient thinks that there is a side-effect. This was proved by conducting a trial involving where in one group was informed about the side-effects while the other was not. The informed group had a precedence rate of 43% for Benign Prostatic Hypertrophy, whereas the uninformed group had just 15%. A few reports also claim that fenasteride has induced depression in the patients, but there is no substantial evidence on the same and further studies have not been conducted on the same. Also, the history of depression among patients have to be considered before arriving at conclusions. (You can read more detailed information on the side effects caused by Nocebo effect)
Finasteride Every Day
For male patients who experience AGA, finasteride is a wonderful option as it can stop hair fall in 9 of 10 incidences. Also, women undergoing post menopause and those who display signs of hyperandrogenism are fount to benefit from this. There are no restrictions involved on when to consume finasteride and there are no allergies or drug interactions involved, as per the several studies that have been conducted. The only aspect that has to be taken care of is if the patients have a history of liver abnormalities.
Although some people may see result in less than 2 months, it is best recommended to use it for 6-9 months before the results start showing up visibly.
Topical Finasteride
Finasteride dissolved in minoxidil soltution to make topical finasteride has the advantage of low cost and enhanced effectiveness compared to finasteride pills. Users are more likely to comply with the daily use. Also it can presumably lower the possible side effects of finasteride by reducing the systemic effect. Read the topical finasteride review here.
Why Is Minoxidil Sulfate A Bad Idea?
Azelaic Acid vs Finasteride for Hair Loss, Which is better?