Why is Minoxidil Sulfate a Bad Idea?
Very often I receive questions asking why I do not recommend minoxidil sulfate in a hair regrowth product.
Perhaps they've read somewhere that says minoxidil sulfate works better than minoxidil.
Here I explain why I do not recommend minoxidil sulfate.
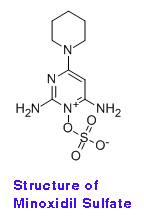
The structure of real minoxidil sulfate is shown in the Figure below. It is the active metabolite that stimulates hair follicles.
Minoxidil applied to the scalp is automatically converted into minoxidil sulfate by sulfotransferase in the scalp (see article 1 below).
Yeah, you got it. First of all, it is unnecessary because minoxidil will be converted to minoxidil sulfate by the enzymes in your body.
Not only is it unnecessary to use minoxidil sulfate, but it is also impractical to include minoxidil sulfate into a solution, because minoxidil sulfate is unstable.
It is hydrolyzed back into minoxidil in a few days (See article 2).
Furthermore, the price of minoxidil sulfate is so prohibitively expensive that nobody can afford to add minoxidil sulfate to hair regrowth solutions.
I am not aware of any supplier that offers a large stock of minoxidil sulfate to make an affordable hair regrowth product.
There are some suppliers that offer very small quantity but for a very high price.
The price was 146 US dollars per 5 milligrams.(May, 2018)
In a 60 ml hair growth solution with12% minoxidil sulfate, it has 7.2grams of minoxidil sulfate, which will cost more than 100,000 US dollars to procure the minoxidil sulfate for one bottle (one month supply).
Obviously, no one would put that much minoxidil sulfate in the solution.
So what happens? The answer is that the manufacturers of hair growth products who they say they have minoxidil sulfate in their products, either never check the raw material they procure, or they just want to fool customers.
Let me show you what I mean.
I bought some minoxidil sulfate a few years back for the purpose of research.
The price was surprisingly lower than minoxidil.
It was hard to believe the price was so low given that the synthesis of the minoxidil sulfate is a lot more complicated than minoxidil.
Therefore I decided to send the sample to a chemical lab to test the material.
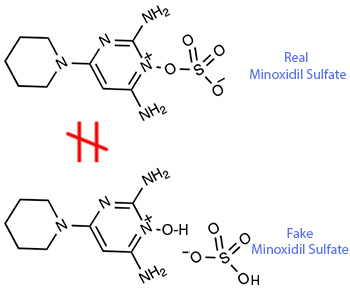
The results showed the material had two molecules in it: (1) minoxidil and (2) sulfuric acid, shown as in main image of this blog.
To make the "minoxidil sulfate" in the main image of this blog is quite easy.
You can get it by simply mixing minoxidil with sulfuric acid.
But the product is not real minoxidil sulfate.
It is simply a mixture of minoxidil and sulfuric acid.
These two molecules are not covalently bonded together.
This is different from the real minoxidil sulfate, in which minoxidil is covalently bonded with sulfuric acid to make one single molecule.
Some people asked me if the "Minoxidil sulfate" they bought online is real or not.
They believe it is real because it dissolves in water so it cannot be minoxidil (minoxidil is not water soluble).
It is true that minoxidil itself is not very soluble in water, but that changes when sulfuric acid is present.
Minoxidil is a weak base, it will be protonated when an acid is presented and it become a positively charged ion and very soluble in water.
That is the exact reason why the fake minoxidil sulfate is very soluble in water because it has both minoxidil and the acid sulfuric acid.
Using the fake "minoxidil sulfate" shown in the main image in a hair growth product will only make the product less effective than minoxidil.
Why? First of all, there is less minoxidil in the product.
Let's say you add 5 grams of the "minoxidil sulfate" to your product, you actually only have 3.2 grams of minoxidil in it.
In addition, sulfuric acid is a strong acid, which will protonize minoxidil and make it positively charged.
It is well known that skin absorbs lipid or non-charged molecules much more effectively than charged molecules.
That means, the presence of sulfuric acid in the product made of "minoxidil sulfate" as shown in Figure 2 will only slow down the absorption.
In summary, a product with minoxidil sulfate does not work any better than minoxidil. If there is any difference, it probably will work less effectively than minoxidil. Minoxidil sulfate is purely a marketing gimmick.
To make minoxidil more effective, one way is to add retinoic acid to minoxidil solution as in Dualgen-5R.
Retinoic acid was shown to double the effectiveness of minoxidil.
Reference:
1. J Invest Dermatol. 1990 Nov;95(5):553-7.Minoxidil sulfate is the active metabolite that stimulates hair follicles.
Buhl AE1, Waldon DJ, Baker CA, Johnson GA.
2. Biochem Pharmacol. 1982 Sep 15;31(18):2949-54.Sulfation of minoxidil by liver sulfotransferase.
Johnson GA, Barsuhn KJ, McCall JM.
Abstract
Lipogaine Review | What Real Users Say About Lipogaine?
Finasteride Frequently Asked Questions & Finasteride Professional Review
Biffy
May 27, 2021What is your take on Spectral.DNC from DS Laboratories, they claim they use minoxidil sulfate? Is it legit?
Bryant
June 1, 2021 AuthorOur opinion on the minoxidil sulfate is stated in the blog. It was written a few year ago. But it is still true today.
chris
January 16, 2020Your price calculation of the minoxidil sulphate formulations based on the sigma reagent is misleading. Pharm-grade minoxidil 99% USP41 is sold by 300~500usd/kg.
Bryant
January 17, 2020 AuthorI appreciate your feedback. The price at Sigma may be on the high end. But we know it is real minoxidil sulfate. We haven't seen any other places that offer real minoxidil sulfate yet.
Matt spaulding
September 19, 2020It's honestly baffling how dumb some people are. The fact that you think you have enough knowledge or understanding to post something like this blows my mind. Reading your article is honestly cringeworthy how moronic you are.
Bryant
September 21, 2020 AuthorWould love to hear what's wrong in the article. Please.
Peter985
January 7, 2020Hi there, there's an error in your fake minoxidil sulfate diagram. Protonated minoxidil wouldn't lose an oxygen atom, the hydrogen should be tagged to the oxygen atom of the N+, while the sulfuric acid fill be deprotonated as you show it. Also, I'd appreciate a discount code for bringing this error to your attention, hehe :D
Bryant
January 8, 2020 Authorhi Peter, Really appreciate your comment. Great catch. We have corrected it. Please contact us for a 10% discount coupon. :)
Stephen M
November 3, 2019The enzyme needed to convert minoxidil into minoxidil sulphate in the scalp is limited. This is why some people respond better than others. The key is to have ample sulfotransferase enzymes in the scalp. There are companies working this angle. If they can increase the enzyme then minoxidil could be a hugely effective drug. Time will tell.
Giannis
May 15, 2019What you say makes a lot of sense. There is study conducted by a group in Brazil confirmed that using minoxidil sulfate is not recommended due to its super instability. Do you have any suggeston on how to make minoxidil more effect?
Bryant
May 15, 2019 AuthorTo make minoxidil more effective, you can add retinol and or azelaic acid like the product Dualgen-5 With PG.